Want to know the difference Between Diamond and Graphite? A Diamond is a stone to be used like a precious gem. It is found in nature in the form of kimberlite stone.
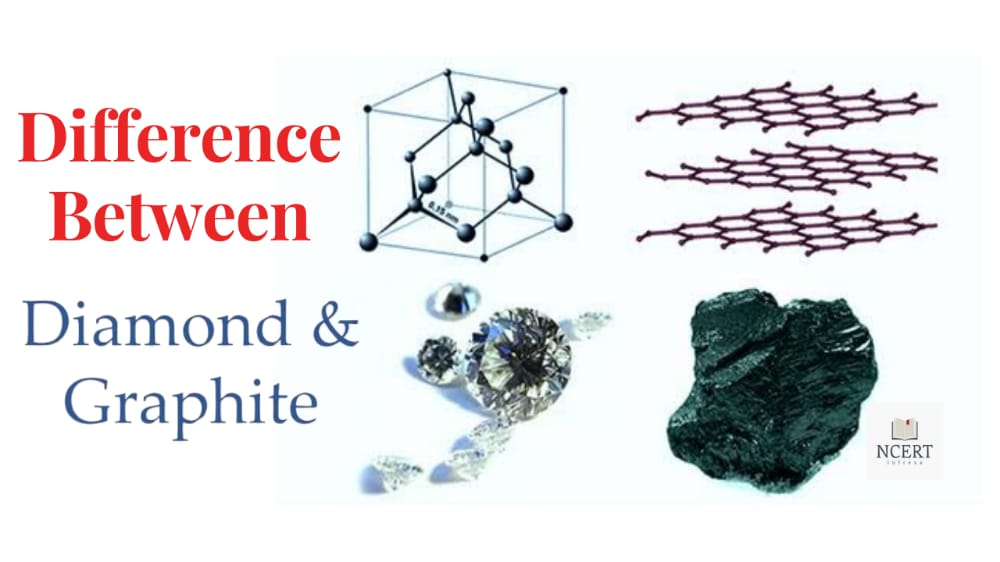
Each carbon atom in a diamond joins tetrahedrally to the other 4 carbon atoms to form a rigid three-dimensional lattice. The specialty of this structure is that directional covalent bonds are present throughout the lattice.
Generally, it is difficult to break the extended covalent bond, and because of that property, diamond is the hardest substance found on Earth.
Difference Between Diamond and Graphite
Diamond and Graphite are dissimilar in terms of density, color, refractive index, and structure.
The difference between diamond and graphite is due to the crystal structure of diamond being different from that of graphite. Below are the main differences between the Diamond and Graphite –
| Sr No. | Diamond | Graphite |
| 1. | The structure of its bones is equi-tetrahedral. | The structure of its veins is in the form of a hexagonal lattice surface. |
| 2. | It is transparent and colorless. | Opaque and ash color. |
| 3. | The relative density is 3.52. | The relative density is 2.2. |
| 4. | It is hard. | It is soft. |
| 5. | Poor conductor of electricity. | Good conductor of electricity. |
| 6. | Bad conductor of heat. | Good conductor of heat. |
| 7. | Used in making jewelry. | It does not make ornaments. |
| 8. | Does not make marks on paper. | Makes a black mark on the paper. |
| 9. | Its refractive index is 2.42 | Its refractive index is 2.7 |
Properties of Diamond
- Pure diamond is always found in the solid state. It is transparent and colorless
- Its density is 3.67, and its refractive index is 2.44.
- It is inert and highly toxic.
- Due to the absence of free electrons, it is a poor conductor of electricity. However, it can conduct heat.
- In pure form, Diamond is transparent to X-rays, but not in impure form. That is why, often, X-rays are used to check the difference between pure and synthetic diamonds.
Uses of Diamond
- As an abrasive for sharpening hard tools (used in making rock drilling machines, in glassware tips, in gemstone cutters).
- Electric light is used in the manufacture of tungsten filaments in lamps.
Graphite
In graphite, each carbon atom is arranged in the form of a hexagonal ring by connecting with three adjacent carbon atoms in the same plane.
Every fourth electron of each carbon atom is free which moves throughout the crystal conductor. Graphite is a good conductor of electricity. Graphite has a two-dimensional sheet-like structure.
Properties of Graphite
- Graphite is soft, ductile, and smooth because of its layered structure. Graphite is so soft and because of this property, is used as a lead pencil to make marks on paper. Hence, Graphite is also known as Black Lead.
- On heating at a very high temperature and maintaining the required pressure conditions in the presence of a suitable catalyst, it turns into a diamond. However, the process is irreversible; therefore, it is not possible to convert diamond into graphite under any circumstances.
Uses of Graphite
- It is used in making electrodes and carbon arcs because it is a good conductor of electricity due to the presence of free electrons.
- Graphite is used in making lead pencils.
- It is used in the manufacture of high-temperature crucibles used for smelting metals.
- Graphite has the property to reflect fast-moving neutrons, and because of this property is used as a moderator in nuclear reactors.
- Its powder is used as a dry lubricant in machines.
- On heating graphite in the presence of a catalyst at very high pressure, it turns into a diamond.